Despite the availability of numerous anti-epileptic drugs (AEDs), about one-third of people with epilepsy continue to have seizures. While some may benefit from resective brain surgery, this is not an option for many patients. The search for alternative therapies to control seizures in this refractory population led researchers to explore deep brain stimulation (DBS) of the anterior nucleus of the thalamus. The value of this intervention was made clear based on the data from the SANTE trial.
In this multicenter, double-blind, randomized trial, 110 adults with refractory partial-onset seizures were implanted with bilateral electrodes in the anterior nuclei of the thalamus. After a one-month post-operative period, half were randomized to receive stimulation while the other half had their stimulators left off. The primary outcome was the change in seizure frequency at the end of the 3-month blinded phase compared to baseline. Safety outcomes included adverse events related to the implantation procedure and stimulation.
During the last month of the blinded phase, the stimulated group experienced a 29% greater reduction in seizure frequency compared to the control group (p=0.002). Complex partial seizures and generalized tonic were also significantly reduced with stimulation. By 2 years, there was a 56% median reduction in seizure frequency, with 54% having at least a 50% reduction. Reported adverse events included implant site pain and paresthesias, but there were no symptomatic brain hemorrhages or infections. Depression and memory impairment were more common in the stimulated group.
After the initial study, researchers continued to follow these patients over the long term. They found that DBS stimulation of the anterior nuclei sustained seizure reduction and an acceptable safety profile over several years. This long-term data was crucial in understanding the durability of the treatment effect and any potential delayed complications.
To summarize, the SANTE (Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy) trial was a landmark study in the field of epilepsy treatment for several reasons:
- The SANTE trial was the first randomized, double-blind, controlled trial to demonstrate the efficacy and safety of deep brain stimulation (DBS) for epilepsy.
- The positive results of the SANTE trial were instrumental in the FDA’s decision to approve the use of anterior thalamic DBS for the treatment of refractory partial-onset epilepsy in 2018.
- The trial introduced a new, less invasive surgical approach to manage seizures in this difficult-to-treat population.
Since its publication, researchers have been exploring other thalamic nuclei as potential targets for DBS in medically refractory epilepsy. For example, the centromedian nucleus (CMN) of the thalamus has been investigated as a potential target due to its widespread cortical projections and role in modulating cortical excitability. Preliminary studies have shown promising results, with significant reductions in seizure frequency and severity in patients with generalized epilepsy syndromes. Other thalamic nuclei, such as the dorsomedial nucleus and the pulvinar, are also being investigated as potential targets. As more research is conducted, it is hoped that targeting these alternative thalamic nuclei may provide additional options for patients with refractory epilepsy who do not respond to anterior thalamic DBS or are not suitable candidates for this intervention.
Feel free to review this Landmark Article here!
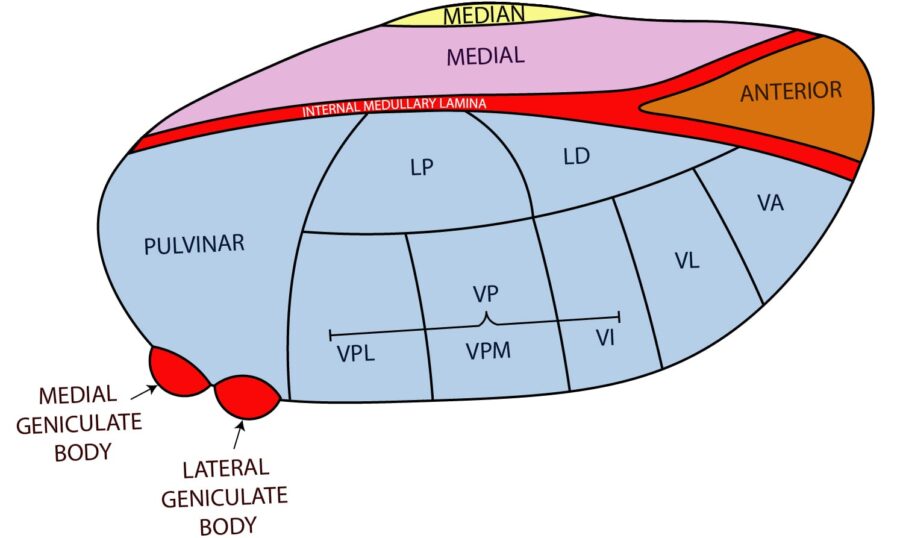
Here is a figure from our site in case you can’t remember your thalamic nuclei!
References:
- Kwan, Patrick, and Martin J. Brodie. “Early identification of refractory epilepsy.” New England Journal of Medicine 342.5 (2000): 314-319.
- Fisher, Robert, et al. “Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy.” Epilepsia 51.5 (2010): 899-908.
- Salanova, Vicenta, et al. “Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy.” Neurology 84.10 (2015): 1017-1025.
- Valentín, Antonio, et al. “Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies.” Epilepsia 54.10 (2013): 1823-1833.
- Velasco, Ana Luisa, et al. “Centromedian nucleus deep brain stimulation for refractory generalized epilepsy.” Epilepsia 58.8 (2017): 1473-1482.
- Yu, Tao, et al. “Thalamic nuclei and their role in epilepsy: A review of literature.” Journal of Neurology, Neurosurgery & Psychiatry 91.8 (2020): 831-839.


